Airborne telescope detects helium hydride ion in space
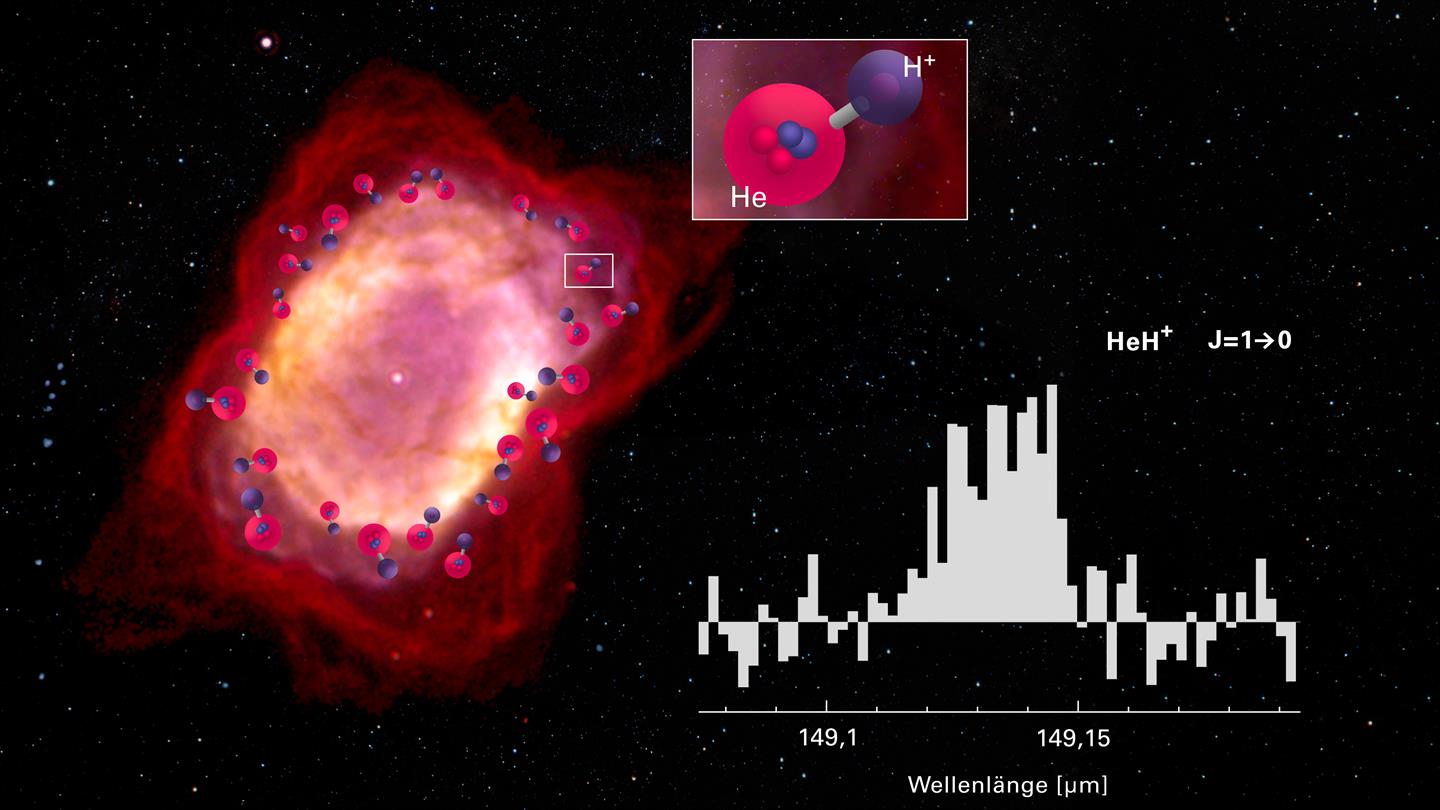
The helium hydride ion HeH+ is a puzzle in and of itself. As a noble gas, helium does not easily bond with other elements. And in the early universe, the selection of elements was much smaller than it is today: the only elements were hydrogen (H), helium (He), and traces of lithium, and only in ionized form, that is, without electrons, which form the basis for chemical bonds. After the big bang, the universe had to cool down first, for a period of approximately 300,000 years, before chemistry could begin.
At a temperature of about 3700 degrees Celsius, the existing atom nuclei recombined with free electrons and thus created the first neutral atoms. According to current popular models, the process began with helium. Hydrogen was still ionized at that time, so helium atoms could combine with free protons to create helium hydride ions HeH+, which therefore became the first molecular compounds in the universe. Later, neutral hydrogen atoms began forming and these reacted with HeH+, creating molecular hydrogen and helium.
This is how science has long imagined the start of chemistry in our universe. But there was one problem: HeH+ ion could be formed in the laboratory, but it was nowhere to be found in space, where it must also be present. The molecule radiates most strongly in a spectral line at a characteristic wavelength of 0.149 mm (corresponding to a frequency of 2.01 terahertz). The Earth’s atmosphere, however, is completely impermeable to wavelengths in this range. So, searching for this molecule became the task for the Stratospheric Observatory for Infrared Astronomy, or SOFIA, which is installed on a modified jumbo jet and is operated jointly by the German Aerospace Center (DLR) and NASA. Equipped with the new GREAT instrument (German Receiver for Astronomy at Terahertz Frequencies) and flying at an altitude of 13 to 14 kilometers (8 to 9 miles), SOFIA could finally detect the long-sought molecule.
“In the past decade, there had been great hopes placed on the Spitzer Space Telescope (NASA, launched 2003) and Herschel Space Observatory (ESA, launched 2009), but neither of these instruments was able to detect this molecule. With SOFIA, we have finally proven that this molecule can actually form in planetary nebulae. Currently there is no other telescope that can make observations at these wavelengths. That will make this platform unique for many more years to come,” said Anke Pagels-Kerp, head of the Space Science department in the DLR Space Administration in Bonn, Germany.